A collaborative group from Tohoku University and Johns Hopkins University have provided valuable insights into the glass transition.
When a liquid is cooled rapidly, it gains viscosity and eventually becomes a rigid solid glass. The point at which it does so is known as the glass transition.
But the exact physics behind the glass transition, and the nature of glass in general, still pose many questions for scientists.
Metallic Glasses (MGs) are highly sought after since they combine the flexibility of plastic with the strength of steel. They are amorphous materials with a disordered atomic structure and exhibit unique and divergent thermodynamic and dynamic characteristics, especially when approaching the glass-transition temperature.
The glass transition in MGs is usually determined by calorimetric and dynamical measurements. The calorimetric glass transition detects the temperature at which specific heat has an abrupt jump, whereas dynamical transition looks at the diverse relaxation responses that emerge with increasing temperature forms.
Generally, the calorimetric glass-transition temperature follows the same trend as the dynamic α-relaxation temperature.
However, the collaborative group discovered that high configuration entropy significantly influences the glass transition of MGs and leads to the decoupling between calorimetric and dynamical glass transitions of high entropy metallic glasses.
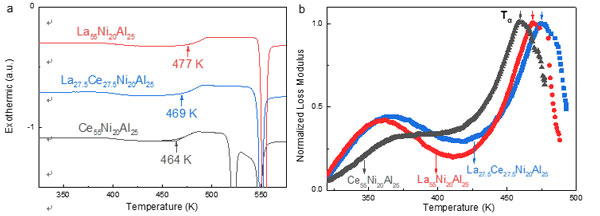
Their study presents a new glass-forming system that uses high configurational entropy, named high entropy metallic glasses (HEMGs).
The group featured Specially Appointed Professor Jing Jiang and Professor Hidemi Kato from the Institute for Materials Research at Tohoku University and Professor Mingwei Chen from Johns Hopkins University.
"We are excited about this discovery and believe this work furthers our understanding of the fundamental mechanism behind the glass transition," said members of the research group.
Publication Details
Title
Authors
Jing Jiang, Zhen Lu, Jie Shen, Takeshi Wada, Hidemi Kato & Mingwei Chen
