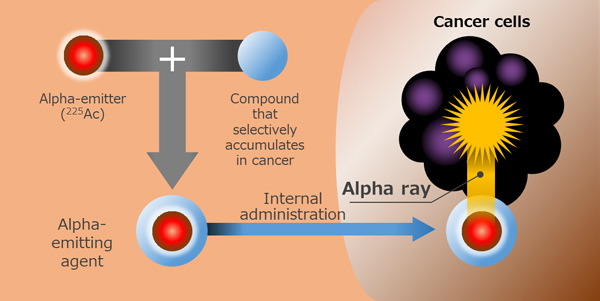
Hitachi, Ltd. (TSE: 6501, “Hitachi”), Tohoku University and Kyoto University have become the world's first to establish technology for the highly efficient and high-quality production of actinium-225 (225Ac), a substance required for a form of radiation therapy known as targeted alpha therapy (TAT). TAT is a new cancer therapy which combines a substance that emits alpha particles which destroy cancer cells with a compound that selectively accumulates in cancer cells. The combined alpha-emitting agent is administered to a patient to attack cancer cells within the body (Figure 1). It is known to be effective against forms of cancers that are difficult to treat with existing methods of treatment, including cancer cells that are spread widely through the body, and its practical applications are eagerly awaited. The three-party team has now established technology that enables production of high-quality 225Ac in an efficient manner without producing impurities, which are usually difficult to separate, by using an electron linear accelerator with radium-226 (226Ra) as a source material.
Hitachi, Tohoku University, and Kyoto University will continue research and development efforts to bring this production technology into commercial use, to help put TAT into clinical practice and ultimately improve cancer patients' quality of life (QoL). In addition, Hitachi is set to start a study to evaluate the applicability of 225Ac produced using this new technology in pharmaceutical products in October 2021 in collaboration with the National Cancer Center Japan. Hitachi makes effort to pursue research and development that promotes the Security & Safety (healthy and comfortable life for each individual) of society.
Types of radiation therapy include external and internal. External radiation uses beams of radiation delivered outside the body to target cancer cells, while internal radiation involves internal delivery of radiation. TAT is a form of treatment in which alpha-emitting agents are administered into the body to selectively target cancer cells, while producing fewer side effects. It is a potentially promising form of treatment especially for cancers that are difficult to treat with existing methods, including advanced cancer where cells are spread widely through the body. Following a report of its high therapeutic effects in patients with metastatic prostate cancer, therapies using 225Ac as an alpha-emitter have been studied for their efficacy and safety in clinical trials across the world. However, the only established method of producing 225Ac that has been one that uses thorium-229, a nuclear material that is difficult to handle, and it produces only a small amount of 225Ac (63 GBq/y). This has posed obstacles to any widespread use of TAT, as 225Ac is not available in a sufficient amount.
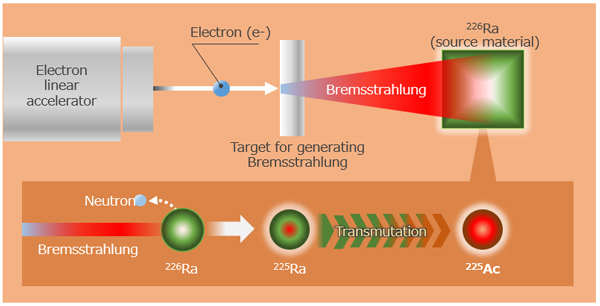
To tackle the situation, Hitachi, Tohoku University’s Research Center for Electron-Photon Science, and Kyoto University’s Institute for Integrated Radiation and Nuclear Science have joined forces to develop a method of producing 225Ac that does not involve nuclear material by applying technologies Hitachi has accumulated in the fields of particle therapy and nuclear power generation. Together, they have successfully established for the first time technology for highly efficient and high-quality production of 225Ac that uses an electron linear accelerator with 226Ra as the source material. This production method involves the use of highly penetrating bremsstrahlung radiation to irradiate 226Ra. In addition to being an efficient production method, it also produces high-quality 225Ac because it does not produce impurities that are difficult to separate (Figure 2).
The team has conducted a proof-of-principle test on the production of 225Ac using a small amount of 226Ra, and collected detailed data on 226Ra's photonuclear reactions. Based on the findings from the test, the team, in a joint research project with the addition of researchers from Tohoku University’s Institute for Materials Research, who own the technology to handle large amounts of radium, has succeeded in producing approximately 370 kBq of 225Ac, an amount that is sufficient for the future evaluation of its applicability in pharmaceutical products. This represents a major step forward for commercial application of this production method, such that the amount of 225Ac currently produced globally in a year (63 GBq/y) can be produced in one day.
Parts of these results will be shared as a Top Rated Oral Presentation at the 34th Annual Congress of the European Association of Nuclear Medicine, which takes place October 20-23, 2021.
Release Details
Release date
October 18, 2021
Press release online (in Japanese)
PDF: 423MB