Summary
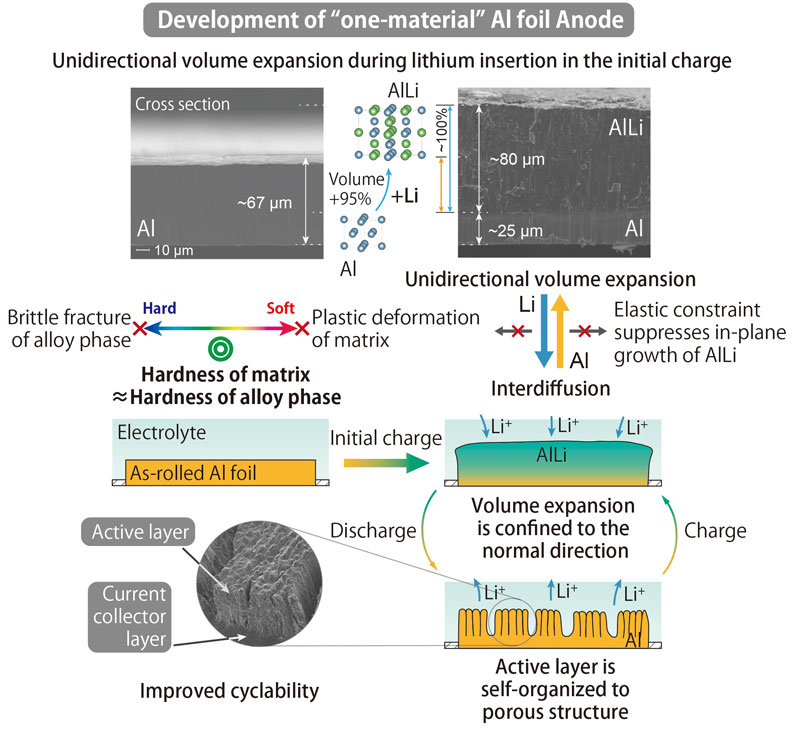
- Discovered that the use of high purity aluminum foil can successfully control the huge volume expansion/contraction of high capacity aluminum anodes during charge/discharge processes, and clarified its mechanism.
- Determined that high purity aluminum foil can be an “integrated anode”, which can replace two components of the conventional graphite anode, i.e., a layered structure of carbon material (that accommodates lithium ions) and copper foil (that functions as a substrate to collect current) as it plays the roles of both components.
- Contributed to higher performance and significant simplification of the battery manufacturing process.
Abstract
Lithium-ion rechargeable batteries consist of four components: a cathode, an anode, an electrolyte, and a separator film. Lithium ions move between the cathode and anode when the battery is charged or discharged; when charging, the anode takes in the lithium ions released by the cathode, and the reverse when discharging. Carbon-based materials are currently the mainstream for anodes. However, the use of silicon or metals such as tin and aluminum, has been considered as promising anode materials for high energy-density batteries because these materials can store three to ten times more lithium ions than the same weight carbon-based materials. Despite the higher capacity for absorbing more lithium ions, the application of metal anodes has been deadlocked because their huge volume changes (two to four times) can destroy the anode’s structure.
Institute for Materials Research, Tohoku University and Sumitomo Chemical will continue their joint research efforts toward practical implementation of the integrated aluminum anode and contribute to the development of a sustainable society.
Publication Details
Authors
Journal
DOI
Online publication date
April 13, 2020
Press release online
PDF: 306KB