Authors:
Keisuke Mukai, Ryuta Kasada, Kiyohiro Yabuuchi, Satoshi Konishi, Jae-Hwan Kim, and Masaru Nakamichi
Journal:
DOI:
Published online:
March 7, 2019
Press release online (Japanese text only):
[PDF: 520KB]
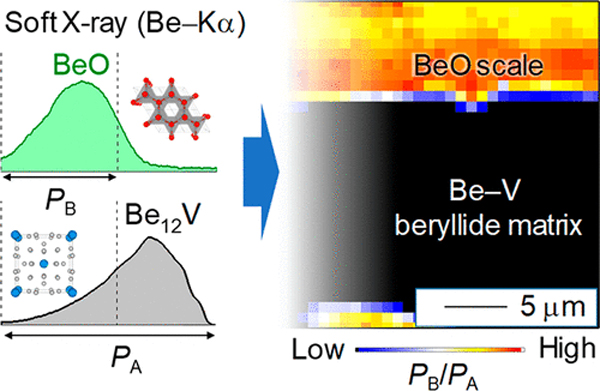
Be-rich intermetallics (beryllides) have gathered wide attentions to be adopted for high temperature environments, such as an advanced neutron multiplier in fusion reactors. This study reveals the valence electron structure of Be12M (M = Ti,V) by using soft X-ray emission spectroscopy with ultrahigh resolution (∼0.22 eV). The Be–Kα spectra from the Be12M phases show significantly lower densities of the occupied states near the Be–K edge than thaose of metallic beryllium. Theoretical calculations indicate that changes in the valence electron structure are derived from the large downward shift of the Fermi level in Be12M, by at least 0.8 eV. Based on the knowledge of the valence electron structures and the chemical shift, chemical state mappings of BeO and Be12V in the oxidized beryllide specimen were successfully obtained. The approach is applicable for visualization and identification of metallic/oxidized phase in light-element compounds by electron microscope. (From Valence Electron and Chemical State Analysis of Be12M (M = Ti, V) Beryllides by Soft X-ray Emission Spectroscopy)
